Page 18 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 18
Original Scientific Article
124
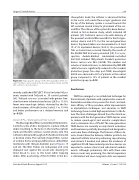
Figure 20: Angiographic image of the Montage Dual Filter Sys- tem. The filters are located in the brachiocephalic trunk and the left common carotid arteries.
recently published DEFLECT III trial included 46 pa- tients treated with TriGuard vs. 39 control patients [48]. TriGuard use was associated with greater free- dom from new ischemic brain lesions (26.9 vs. 11.5%), fewer new neurologic deficits detected by the Na- tional Institutes of Health Stroke Scale (3.1 vs. 15.4%) and better performance on a delayed memory task (p=0.028).
Claret CE Pro / Montage Dual Filter System
The Montage Dual Filter System (Claret Medical Inc., Santa Rosa, CA, USA) is designed to capture embolic debris travelling to the brain in the brachiocephalic trunk and the left common carotid arteries [46]. The catheter is delivered through a 6 Fr sheath via the radial or brachial artery. The conically shaped filters consist of a nitinol frame and polyurethane laser-drilled filter membrane with 140 μm-diameter pores (Figures 19 and 20). The filter frames are radiopaque and once deployed seal against the vessel wall, allowing fil- tered blood to pass to the brain while trapping debris. After positioning of the first filter in the bra-
chiocephalic trunk, the catheter is advanced further in the aortic arch under fluoroscopic guidance and the tip of the delivery system is curved towards the left common carotid artery for placement of the sec- ond filter. The safe use of the system has been demon- strated in first-in-human study, which included 40 patients [49]. Technical success rate with delivery of the proximal and distal filter was 60% for the first gen- eration device and 87% for the second-generation device. Captured debris was documented in at least 19 of 35 implanted devices (54.3%). No procedural TIAs or strokes have occurred. Recently, the results of the CLEAN-TAVI trial were presented [50]. It is a pro- spective, double-blinded, randomized-controlled trial that included 100 patients. Cerebral protection device success was 96% (48/50). The number and volume of cerebral lesions as determined by DW-MRI subtraction was significantly reduced in the cerebral protection group. Two days post TAVR, neurological deficit was observed in 28% of patients in the control group compared to 13% of patients in the cerebral protection group (p=0.08).
Conclusions
TAVR has emerged as an established technique for the treatment of patients with symptomatic severe AS. Cumulative evidence has proven the short- and mid- term efficacy of this procedure, while improvements in implantation techniques and advances in TAVR technology have created high expectations for the fu- ture. The main challenges derived from the clinical ex- perience with the first-generation TAVR devices were to reduce neurological and vascular complications and to minimize rates of PV-AR. The new-generation TAVR devices are currently in early clinical evaluation and have been specifically developed and designed to overcome these challenges. The features of these de- vices should allow the delivery catheter profile to be reduced, facilitate accurate positioning, repositioning and retrieval if needed, and reduce the incidence of significant PV-AR. New cerebral protection devices are expected to reduce clinical and sub-clinical embolic events. Although preliminary data with these new devices seem very promising, the clinical experience is still limited and more long-term data are required.
Structural Heart Disease, August 2015
Volume 1, Issue 3: 112-126