Page 7 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 7
113
Original Scientific Article
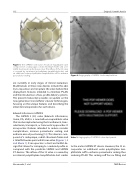
Figure 1: The SAPIEN 3 (S3) valve. A balloon-expandable valve composed of a radiopaque, cobalt chromium frame and a tri- leaflet bovine pericardial tissue valve. The inflow of the S3 valve is covered by an internal polyethylene terephthalate skirt and an additional outer polyethylene terephthalate cuff to enhance paravalvular sealing.
are currently in early stages of clinical evaluation. Modifications in these new devices include the abil- ity to reposition and recapture the valve before final deployment, features intended to minimize PV-AR, and the introduction of low- profile delivery systems. The present manuscript provides an update on the new-generation transcatheter valvular technologies, focusing on the unique features and describing the initial clinical experience for each device.
Edwards Lifesciences SAPIEN 3
The SAPIEN 3 (S3) valve (Edwards Lifesciences, Irvine, CA, USA) is a new balloon-expandable valve that can be implanted using the transfemoral, trans- subclavian, transapical, or transaortic approaches. It incorporates features intended to reduce vascular complications, increase paravalvular sealing, and enhance ease of positioning [12]. This device is com- posed of a radiopaque, cobalt chromium frame and a trileaflet bovine pericardial tissue valve (Figures 1, 2 and Movie 1). It incorporates a stent and leaflet de- sign that allows for crimping to a reduced profile as compared with the predicate SAPIEN and SAPIEN XT devices. The inflow of the S3 valve is covered by an internal polyethylene terephthalate skirt similar
Figure 2: Angiography of SAPIEN 3 valve implantation.
Video 1: Angiography of SAPIEN 3 valve deployment.
to the earlier SAPIEN XT device. However, the S3 in- corporates an additional outer polyethylene tere- phthalate cuff to enhance paravalvular sealing thus reducing PV-AR. This sealing cuff has no filling and
Abramowitz, Y. et al.
TAVR Devices