Page 19 - Journal of Structural Heart Disease Volume 2, Issue 6
P. 19
Meeting Abstracts
246
2 to 15). Of 26 VSDs; there were 6 midmuscular, one apical muscular and 19 PM VSDs. Muscular VSDs were closed by muscular occluders but PM VSDs were closed by 10ADO 1,3 ADOII and 6 muscular occluders. Three patients underwent successfully combined transcatheter intervention; one patients underwent ASD closure with PM VSD closure, the second patient underwent PDA closure with PMVSD closure and the third patient underwent balloon pulmonary valvuloplasty with midmuscu- lar VSD closure . The device embolized in one case to right pulmonary artery (6.3%). Retrieval attempt was unsuccessful. The VSD occlusion rate was 69 % at completion of the procedure, rising up to 83% at dis- charge and 97 % during follow-up. Small residual shunts were seen at completion of the procedure, but they disappeared during follow-up in all except one patient. The median follow-up period was 8 months (range 1 to 15 months). Complete atrioventricular block (CAVB), major complication or death was not observed in our study.
Conclusions: Percutaneous closure of muscular or PM VSDs with di erent Amplatzer device occluders is safe and feasible with good mid-term outcomes , but large numbers of patients are required.
#0014
INTRAVASCULAR ULTRASOUND IN PULMONARY VEIN STENOSIS INTERVENTIONS
Michael D. Seckeler1, Alicia Hudson2, Kwan Lee3 1Department of Pediatrics (Cardiology), Banner University Medical Center - Tucson/University of Arizona, Tucson, AZ, USA 2University of Arizona College of Medicine, Tucson, AZ, USA 3Department of Cardiology, Banner University Medical Center - Tucson/University of Arizona, Tucson, AZ, USA
Background: Pulmonary vein stenosis (PVS) is an aggressive disease with high rates of morbidity and mortality. Surgical and catheter interven- tional approaches have yielded modest success, at best. Re nements in catheter intervention could potentially improve outcomes.
Methods: Single-center, retrospective review of patients with PVS undergoing cath from 3/2015 – 8/2016. As part of the diagnostic cath, the left atrium was entered via an existing septal defect or by transseptal puncture. Systemic heparinization was provided to main- tain ACT>250. Intravascular ultrasound (IVUS) of the pulmonary veins was performed using an Eagle Eye® Platinum IVUS catheter (Volcano Corp, Rancho Cordova, CA).
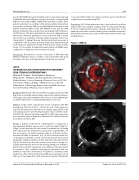
Results: 3 patients underwent 4 catheterizations (1 diagnostic, 3 interventional). Median age was 2.2y (0.7-47.5y), weight 9.9kg (7.3- 61kg). For the interventional caths, mean PV gradient was 10 mmHg with reduction by 8.8 mmHg. Two patients had congenital PVS, one was post-repair of Scimitar syndrome with an obstructed pulmo- nary venous ba e. For patient 1 (Fig. 1a), IVUS showed interval ves- sel growth (arrowheads) around a previously placed stent (arrow) allowing for redilation with good stent apposition after intervention (Fig 1b). On follow-up cath, stenosis was seen in areas no longer cov- ered by stent material, due to foreshortening during prior redilation, which improved with angioplasty and additional stent placement. For patient 2, IVUS con rmed long-segment hypoplasia that was unlikely to respond to intervention. For patient 3, narrowing in the midportion of the obstructed Scimitar ba e was seen (Fig. 2a, arrow) with good stent apposition after intervention (Fig 2b). All patients
recovered well from their procedures and there were no thrombotic complications from performing IVUS.
Conclusions: IVUS of the pulmonary veins is safe and easy to perform and provides very detailed imaging of PVS to help guide therapy. For those requiring intervention, adequate stent apposition to the pulmonary vein walls as well as limiting vessel overdilation may min- imize future instent stenosis and need for reintervention in this chal- lenging disease.
Figure 1 (#0014).
A
B
Journal of Structural Heart Disease, December 2016
Volume 2, Issue 6:241-306