Page 10 - Journal of Structural Heart Disease Volume 3, Issue 1
P. 10
3 Original Scienti c Article
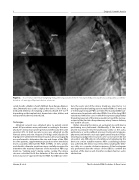
Figure 2. In a 15-year-old female weighing 54 kg, the long variant of the 5-7 Occlutech Duct Occluder was adequately accommo- dated in a 3-mm type D patent ductus arteriosus.
cated sheath, a Mullins sheath (William Cook Europe, Bjæver- skov, Denmark) was used to deploy the device. Since then, a dedicated delivery set including a delivery sheath (6-F to 9-F depending on the implant size), hemostasis valve, dilator, and transparent loader was introduced.
Technique
Informed consent was obtained prior to patient enroll- ment. All procedures were performed according to the man- ufacturer’s instructions under general anesthesia by the same operator (ZS). In brief, vascular access was obtained via the femoral artery and vein. Heparin (50I U/kg) and Cefazolin (30 mg/kg) were administered intravenously. Lateral aortography was performed to determine the size and shape of the PDA. In some patients, supplementary projections were needed to more accurately delineate the PDA. In PDAs >3 mm, systolic and diastolic diameter variations were carefully measured to determine the maximal diameter of the narrowest PDA seg- ment (i.e., “landing zone”) during cardiac cycling. The device size was calculated to t the diameter of the area where the device was to be “squeezed” into the PDA. For ODO size selec-
tion, the aortic end of the device shank was sized to be 1–2 mm larger than the landing zone in smaller PDAs (<3 mm) and 2–3 mm larger in larger PDAs (≥3 mm). The standard ODO vari- ant was used in patients with short PDAs. Use of the long ODO variant was limited to cases in which the operator judged that the pulmonary part of the device would not reach the narrow- est part of the duct into the pulmonary artery. Venous delivery was used in all cases.
Before releasing the device, an aortogram to verify device positioning was performed. Additionally, if the device ap- peared to protrude into the pulmonary artery or the aorta, pulmonary or aortic pullback pressure tracing and angiogra- phy were performed to rule out signi cant obstruction. Repo- sitioning was judged necessary in some cases, but we did not encounter any di culty in resheathing and redeploying the device in a more suitable manner. After a satisfying position was achieved, the device was released by rotating the deliv- ery cable in a counter-clockwise manner. A nal aortogram was performed after the release of the ODO to con rm the position of the device and check for residual shunt or aortic obstruction.
Hanna, N. et al.
The Occlutech PDA Occluder : A Case Series