Page 40 - Journal of Structural Heart Disease Volume 5, Issue 6
P. 40
Case Report
264
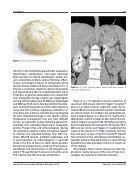
Figure 1. The Occlutech® ASD Ocludder.
(ASO) [4, 5], the method has gained wider acceptance. Nevertheless, complications have been described. More common are device embolization, cardiac ero- sion, atrioventricular block, atrial arrhythmias, inflam- matory and allergic reactions to components of the devices (mainly nickel) and device thrombosis [6-11]. The last is commonly related to device implantation and should decline after its endothelialization (up to 6 months). In general, adult patients are treated with dual antiplatelet therapy (Aspirin plus Clopidogrel), starting with a loading dose of 300mg of Clopidogrel and 300mg of ASA two to four days before the proce- dure, and continuously for six months after treatment, using data from coronary angioplasty protocols [12], since there are no trials to date that have investigated the best antiplatelet therapy in this specific setting. Preoperative management may vary from different centers, but generally involve collecting general he- matologic tests (complete blood count, coagulation profile), Thorax X-Ray and echocardiogram. During the procedure, patients receive intravenous heparin to maintain the Activated Clotting Time (ACT) be- tween 200-250 seconds, antibiotic prophylaxis, and in post-procedure an echocardiogram is generally made in the first 24 hours to check device position, presence of residual shunts, evidence of new pericar- dial effusion and valve function. In the long term evo- lution, TTE is performed in one and six months, and a TOE is performed after one year of treatment.
Figure 2. CT Scan showing apical splenic infarction (arrow), 6 years after ASD closure.
Pedra et al. [13] reported mid-term outcomes of secundum ASD closure with the Figulla® Occlutech® device in an observational, single-arm study that in- cluded 200 consecutive patients treated in two Brazil- ian referral centers. For children, the antiplatelet pro- tocol included Aspirin in a dose of 3-5 mg/Kg (max: 200mg/day), started a couple of days before the pro- cedure. Heparin was given (100-150 IU/Kg) during the device implantation to maintain the ACT greater than 200 seconds. Anmar and Hegazy [14] published the results of the closure of 17 ASDs in patients with less than two years of age, using the Occlutech® Figulla® Flex occluder. For this subsetting (small children), an- tiplatelet protocol included Aspirin in a dose of 5mg/ Kg before the index procedure and for six months af- ter the implant.
Nevertheless, there is scarce data on very late com- plications, mainly because of the lack of trials to in- vestigate very long term treatment evolution.
Journal of Structural Heart Disease, December 2019
Volume 5, Issue 6:263-267