Page 10 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 10
Original Scientific Article
116
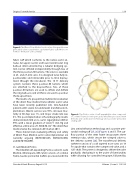
Figure 5: The Direct Flow Medical aortic valve. A tricuspid bovine pericardial valve is attached to a polyester fabric cuff which con- forms to the native aortic annulus.
fabric cuff which conforms to the native aortic an- nulus. An upper (aortic) and lower (ventricular) ring balloon interconnected by a tubular bridging sys- tem can be inflated independently through two of the three position-fill lumens. The valve is available in 25- and 27-mm sizes. It is designed to be fully re- positionable and retrievable prior to final deploy- ment through the introducer. The 18 Fr delivery system contains three position-fill lumens which are attached to the bioprosthesis. Two of these position-fill lumens are used to inflate and deflate the ring balloons and all three are used to position the bioprosthesis.
The results of a prospective multicenter evaluation of the direct flow medical transcatheter aortic valve have been recently published [24]. One-hundred patients with severe AS underwent transfemoral im- plantations. Device success was 93%, all-cause mor- tality at 30 days was 1%, and major stroke rate was 4%. The post-implantation echocardiography results demonstrated mild or no aortic regurgitation (AR) in 99% with a mean gradient of 12.6±7.1 mm Hg and effective orifice area of 1.50±0.56 cm2. The direct flow medical valve has received a CE mark at 2013.
Three clinical trials evaluating efficacy and safety of Direct Flow Medical aortic valve implantation are currently ongoing (NCT01845285, NCT02163850, and NCT01932099).
St. Jude Medical Portico
The trileaflet self-expanding Portico valve (St. Jude Medical, Minneapolis, MN, USA) consists of a nitinol frame, bovine pericardial leaflets processed with the
Figure 6: The Portico valve. A self-expandable valve composed of a nitinol frame, bovine pericardial trileaflets processed with the Linx anti-calcification technology and a porcine pericardial sealing cuff.
Linx anticalcification technology and a porcine peri- cardial sealing cuff [25, 26] (Figures 6 and 7). The out- flow portion of the stent frame incorporates three retention tabs, which secure the crimped valve to the delivery system [26]. The transfemoral delivery catheter consists of a soft tapered nose cone, an 18 Fr capsule that contains the compressed valve, and a 12Fr shaft. The system is designed to deliver the valve gradually, deploying it to the point of functionality while allowing for controlled recapture, followed by
Structural Heart Disease, August 2015
Volume 1, Issue 3: 112-126