Page 11 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 11
117
Original Scientific Article
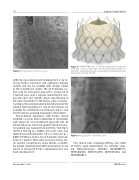
Figure 7: Angiography of a Portico valve.
either by repositioning and redeployment or by re- moval. Portico transaortic and subclavian delivery systems will also be available with designs similar to the transfemoral system. The 24 Fr delivery sys- tem used for transapical approach is composed of a tapered nose cone, a capsule containing the com- pressed valve and similarly allows repositioning of the valve if needed [27]. The Portico valve is sized ac- cording to the nominal external stent diameter at the valvular level. Currently, 23- and 25-mm devices are available for commercial use in Europe, and 27- and 29-mm devices are being evaluated in clinical trials.
First-in-human experience with Portico device included a 23-mm device implanted in 10 patients with severe AS via transfemoral approach [26]. At 30-day follow-up, echocardiographic mean transaor- tic gradient was reduced from 44.9±16.7 mm Hg to 10.9±3.8 mm Hg (p < 0.001), and aortic valve area (AVA) increased from 0.6±0.1 cm2 to 1.3±0.2 cm2 (p < 0.001). PV-AR was mild or less in 9 patients and mod- erate in 1 patient. There were no major strokes, ma- jor vascular complications, major bleeds, or deaths. No patient required pacemaker implantation. A case report of transapical Portico implantation has also been described [27].
Figure 8: The CENTERA valve. A self-expandable ultra-low-profile valve that consists of three bovine pericardial tissue leaflets at- tached to a nitinol frame with a polyethylene terephthalate skirt intended to minimize PV-AR.
Figure 9: Angiography of a Centera valve.
Five clinical trials evaluating efficacy and safety of Portico valve implantation are currently ongo- ing (ClinicalTrials.gov Identifier: NCT02000115, NCT01802788, NCT01742598, NCT01493284, and NCT02088021).
Abramowitz, Y. et al.
TAVR Devices