Page 13 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 13
119
Original Scientific Article
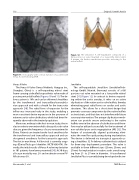
Figure 11: Aortography with contrast injection of a patient with Venus A valve following TAVR.
Venus A Valve
The Venus A Valve (Venus Medtech, Hangzou Inc., Shanghai, China) is a self-expanding nitinol stent frame carrying a trileaflet bioprosthetic valve made of porcine pericardial leaflets (Figures 10 and 11). The de- livery system is 18Fr and can be delivered sheathless by the transfemoral and transaxillary/transsubcla- vian approach and with a sheath for the transaortic approach [30]. The radial force of expansion for the inflow was increased early in the study, enabling a more consistent device expansion in the presence of extreme aortic valve calcification, which had been fre- quently observed in the treated population.
Moreover, midway in the first-in-man study, the in- clusion criteria were extended to bicuspid aortic valve disease, given the frequency of cases encountered in China. Patients are treated under local anesthesia for the transfemoral and transaxillary approach and un- der general anesthesia for the transaortic approach. The first in-man Venus A-Valve trial is currently ongo- ing (ClinicalTrials.gov Identifier: NCT01683474). Re- cently, the initial results of Venus A valve implantation in 101 patients have been presented [30]. At 30-days, all-cause mortality was 2% and moderate-severe PV- AR rate was 6%.
Figure 12: The JenaValve. A self-expandable composed of a full porcine root valve mounted on a low-profile nitinol stent. A unique clip fixation mechanism provides anchoring to the native leaflets.
JenaValve
The self-expandable JenaValve (JenaValveTech- nology GmbH, Munich, Germany) consists of a full porcine root valve mounted on a low-profile nitinol stent [31] (Figure 12). In contrast to devices expand- ing within the aortic annulus, it relies on an active clip fixation of the native aortic valve leaflets, thereby eliminating great radial forces on cardiac and aortic structures. This allows for a short stent design that prevents coronary compromise by the native leaflets or stent struts, and that does not interfere with future coronary intervention. The unique clip fixation mech- anism can provide secure anchoring to the native leaflets even in the absence of calcification and there- fore may be utilized successfully for the treatment of non-calcified pure aortic regurgitation (AR) [32]. The feature of anatomically aligned positioning elimi- nates the need for rapid pacing during implantation. The device is delivered via transapical approach us- ing a sheathless 32 Fr delivery catheter that is utilized for three-step deployment procedure. The valve is available in three different sizes (23mm, 25mm, and 27mm) for implantation in native aortic annuli rang- ing from 21- to 27-mm in diameter. A transfemoral JenaValve Plus is currently being developed with sim-
Abramowitz, Y. et al.
TAVR Devices