Page 14 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 14
Original Scientific Article
120
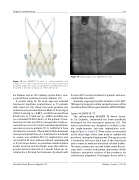
Figure 13: The ACURATE TA valve. A self-expandable valve composed of a nitinol stent frame and a biological tissue valve mounted within the stent. A polyethylene terephthalate skirt is mounted at the intra-annular part of the stent body.
ilar features and an 18 Fr delivery system that is com- posed of three combined coaxial catheters [33].
A pivotal study for CE mark approval included transapical JenaValve implantations in 73 patients with severe AS [34]. Mean transaortic gradient was reduced post-procedurally from 40.6±15.9 mm Hg to 10.0±7.2 mm Hg, (p < 0.001), and AVA increased from 0.7±0.2 cm2 to 1.7±0.6 cm2 (p < 0.001) and there was no or minimal PV-AR in 86.4% of the patients. Proce- duralsuccessratewas89.6%,perioperativestrokeoc- curred in two cases (3%) and pacemaker implantation was necessary in six patients (9.1%). Seiffert et al. have described a case series of five patients that underwent transapical implantation of a JenaValve for moderate to severe, non-calcified AR [32]. Implantation was successful in all cases without relevant remaining AR or AS. No major device- or procedure-related adverse events occurred and all patients were alive with im- proved exercise tolerance at 3-month follow-up. Je- naValve has a CE mark for treatment of patients with
Figure 14: Angiography of an ACURATE TA valve.
AS since 2011 and for treatment of patients with non- calcified AR since 2013.
Currently ongoing trial with JenaValve is the JUPI- TER registry (long-term safety and performance of the JenaValve; ClinicalTrials.gov Identifier: NCT01598844).
Symetis ACURATE TA / TF
The self-expanding ACURATE TA device (Syme- tis SA, Ecublens, Switzerland) has been specifically developed for the transapical approach [35]. The nitinol stent frame was designed to facilitate a sim- ple single-operator two-step implantation tech- nique (Figures 13 and 14). Three arches are mounted at the distal edge of the stent body to stabilize the prosthesis during final deployment. The upper crown is formed by the most distal part of the stent body and is meant to embrace the native calcified leaflets. The stent commissures are well visible under fluoros- copy with a circular radiopaque appearance which facilitates anatomical rotation of the prosthesis for commissural alignment. A biological tissue valve is
Structural Heart Disease, August 2015
Volume 1, Issue 3: 112-126