Page 23 - Journal of Structural Heart Disease Volume 2, Issue 1
P. 23
17
Original Research Report
Video 5.
Slide # 19:
ICE: Intracardiac echocardiography
TTE: Transthoracic echocardiography TEE: Transesophageal echocardiography
Slide # 25:
Stop- ow technique: The sizing balloon is placed across the ASD and in ated until there is stoppage of ow across the defect on color- ow Doppler imaging. The maximum width of the balloon is then measured on TTE/TEE/ICE as well as uoroscopy.
Waist measurement technique: The sizing balloon is placed across the ASD and in ated until there is a waist formation noted along both the margins of the balloon on uoroscopy. This waist is then measured on uoroscopy.
Slide # 26:
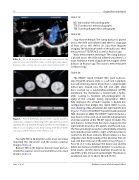
The HELEXR Septal Occluder (W.L: Gore & Associ- ates, Flagsta , Arizona, USA) is a soft and compliant, non-self-centering device made from a single-length nitinol wire shaped into the left and right atrial discs covered by a polytetra ouroethylene (ePTFE) membrane. The membrane is treated with a hydro- philic coating to facilitate echocardiographic im- aging of the occluder during implantation. When fully deployed, the occluder assumes a double disc con guration that bridges the septal defect to pre- vent shunting of blood between the right and left atria (Figure 1: Panel A). The HELEXR Septal Occluder received FDA clearance in 2006. The HELEXR Septal Occluder, a new device, is the result of an extended development and improvement of the HELEXR Septal Occluder. The wire frame is formed from ve wires shaped into the right and left atrial discs, the eyelets, and the lock loop. The ve-wire design provides conformability, allowing each individual wire within a right or left atrial disc to conform to the heart anatomy. Device release is a two staged process, rstly locking of the device by the lock loop and then removal of the retrieval cord (Figure 1: Panel B). A 2:1 ratio between the device size and the defect size “balloon-stretched diameter” is used for op- timal results, and the device diameter should not ex- ceed 90% of the measured septal length. The device is available in sizes of 15, 20, 25, 30, and 35 mm.
TEE at 90 degrees (bicaval view) demonstrates the superior vena caval and inferior vena caval margins. View supple- mentary video at http://dx.doi.org/10.12945/j.jshd.2016.007.14.
Figure 1.
Panel A. When fully deployed, the occluder assumes a double disc con guration that bridges the septal defect to prevent shunting of blood between the right and left atria. Panel B. Device release is a two staged process, rstly locking of the device by the lock loop and then removal of the retrieval cord.
Top right: TEE at 40 degrees (aortic short axis view), depicting the retroaortic and the postero-superior margins (Video 4).
Bottom: TEE at 90 degrees (bicaval view) demon- strates the superior vena caval and inferior vena caval margins (Video 5).
Jain, S. et al.
Catheter closure of atrial septal defect