Page 32 - Journal of Structural Heart Disease Volume 5, Issue 5
P. 32
225 New Technology
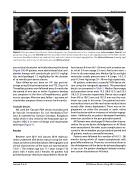
Figure 4. Post-procedure Trans-thoracic Echocardiography (an illustrated case) in four chamber view (color compare, Panel A) and parasternal long axis view (Panel B) after the procedure which demonstrated the secure, well aligned position of the ADO I like de- vice across the defect without any residual shunt and aortic, mitral or tricuspid regurgitations. The white solid arrow showing right ventricular disc and no disc on left ventrcular side (White asterisk).
any residual shunt prior to finally releasing the device (Figure 3D) All patients were administered dual anti- platelet therapy with acetylsalicylic acid 3-5 mg/kg/ day and clopidogrel 1-2 mg/Kg/day for the duration of six months post device closure.
Close follow-up was done on 10th day post-dis- charge using Clinical examination and TTE. (Figure 4) Thereafter, patients were followed every 3 months for the period of one year or earlier if patients develop any symptoms in the form of breathlessness, giddi- ness or syncope. After one year, follow – ups were ad- vised either symptom driven or every 4 to 6 months.
Occluder devices
We used the ‘Cocoon’ PDA device [manufactured by Vascular lnnvovations Co. Ltd. Nonthaburi,Thai- land & marketed by Vascular Concepts, Bangalore, India] which is very similar to the Amplatzer duct oc- cluder I (ADO I) in terms of design and implantation technique (Figure 1).
Results
Between June 2015 and January 2018, eight pa- tients underwent VSD device closure using the tech- nique and device described above. Demographic and clinical characteristics of the cases are summarized in Table 1. The median age was 17.1 years (range 5-32 years) with 3 males and 5 females. All patients had perimembranous ventricular septal defects. Mean de-
fect size was 6.6 mm (4.5 - 8.6 mm), with a median aor- tic rim of 3.4 mm (range 2-5 mm). Aortic rims were < 5 mm in all cases except one. Median Qp/Qs and right ventricular systolic pressure were 1.8 (range: 1.6–2.1) and 41.3 mm Hg (range: 33– 50 mm Hg) respectively.
All patients underwent successful VSD device clo- sure using the retrograde approach. The procedural details are presented in Table 2. Median fluoroscopy and procedure times were 13.3 (10.6-15.7) and 23.5 (18.2-27.2) minutes respectively. Device sizes ranged from 4/6 to 10/12 mm and 10/12 mm was the max- imum size used in this series. One patient had mini- mal residual shunt and the rest had no residual shunt acutely after device deployment. There was no im- pingement on either the tricuspid or aortic valves from the device with no instances of valvular regurgi- tation. Additionally no patient developed hemolytic anemia or jaundice in the post procedure period.
All Patients were discharged 24 hours after the pro- cedure. No acute device dislodgements or AV block were encountered. No other acute complications oc- curred in the immediate post procedural period and all patients made an uneventful recovery.
Patients were followed for a median of 18 (12 - 28) months during which all stayed asymptomatic with good effort tolerance. There was no residual shunt or late dislodgement of the device by echocardiography in any case. No patient developed delayed conduc- tion system disturbance or AV block.
Jariwala P. et al.
VSD Device Closure Using ADO I Like Device.