Page 36 - Journal of Structural Heart Disease - Volume 1 Issue 1
P. 36
Original Research Article
30
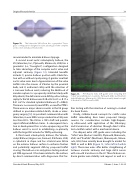
Figure 12. The Intervalve V8 balloon has a geometric “hour- glass” configuration designed to take advantage of the complex aortic valve and adjacent anatomy.
recommended to minimize balloon slippage.
A second novel aortic valvuloplasty balloon, the V8 (Intervalve, Inc., Plymouth, Minnesota, USA) has a geometric (i.e., “hourglass”) configuration designed to take advantage of the complex aortic valve and adjacent anatomy (Figure 12). Intended benefits include: 1) precise balloon position with stable fixa- tion with or without rapid pacing, 2) greater resultant aortic valve areas due to hyperextension of the valve leaflets into the sinuses of Valsalva by the proximal bulb, and 3) enhanced safety with the retention of a narrower balloon waist, reducing the likelihood of annular rupture. In a propensity-matched study with 40 patients, the delta increase in AVA by echocardiog- raphy for the V8 balloon was 0.30 ± 0.23 cm2 vs. 0.17 ± 0.21 cm2 for standard cylindrical balloons (P = 0.063). There was no severe AI, new IVCD’s, or need for PPM’s. There were no major adverse events in the V8 group defined as procedure-related death, stroke or emer- gency surgery [12]. As per direct communication with Intervalve, in over 400 cases procedural mortality was less than 0.5%. The V8 has a 10Fr shaft and permits rapid inflation/deflation times. A subsequent itera- tion in development has a radio-opaque ring on the balloon waist to assist in establishing co-planarity
with the target AV annulus for TAVR positioning.
A third novel valvuloplasty balloon, the Valvulo- sculpt Balloon (Angioscore, Fremont, California, USA) is cylindrical in shape and has a helical wire lattice on the exterior balloon surface to enhance fixation and potentially augment AVA by proposed leaflet scoring. This balloon is in early phase testing and not yet FDA approved. It has been related to the authors by direct communication with Angioscore that fur-
Figure 13.
Pre-shaped, extra stiff, guide wires including the “Safari” wire (left-Boston Scientific, Plymouth, MN) and “Confida” (right-Medtronic, Minneapolis, MN) have recently been brought to market for TAVR as well as BAV.
ther testing with the intention of coming to market has been frozen.
Finally, catheter-based concepts for calcific valve leaflet remodeling have been proposed. Energy sources for consideration include high-frequen- cy ultrasound, with application of the lithotripsy, and transmission of vibration through direct cathe- ter-to-leaflet contact with a mechanical device.
Pre-shaped, extra stiff, guide wires including the “Safari” wire (Boston Scientific, Plymouth, Minnesota, USA) and “Confida” (Medtronic, Minneapolis, Minne- sota, USA) have recently been brought to market for TAVR as well as BAV (Figure 13, Safari left & Confida right). These new wires offer better straightening of tortuous vascular anatomy and large distal curves with smooth transitions in the LV. These features en- hance greater wire stability and support as well as a
Structural Heart Disease, May 2015
Volume 1, Issue 1: 20-32