Page 25 - Journal of Structural Heart Disease Volume 1, Issue 3
P. 25
131
Original Scientific Article
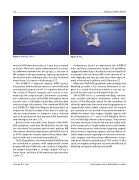
Figure 3: MitraClip device
ment in NYHA functional class at 1 year was sustained at 4 years. The 4-year results demonstrated no mortal- ity difference between the two groups, a low rate of MV surgery in the percutaneous repair group beyond the first 6 months of therapy, and a low rate of adverse events from 1 to 4 years in both groups [17].
The EVEREST II high-risk registry (HRR) includ- ed patients with moderate-severe or severe MR with an estimated surgical risk of 12% or greater (based on the Society of Thoracic Surgeons risk score or as esti- mated by the surgical team). Enrolment of patients has continued as part of the REALISM registry which has two arms: 1 with high-risk patients and the other with non-high risk patients, The combined REALSIM and EVEREST II High Risk Registry demonstrated an impressive 30 day mortality of less than 5% with sig- nificant improvement in symptom status, reduced rate of hospitalization and improved left ventricular remodeling at one year [15].
Based on the outcomes from Everest II the AHA/ ACC guidelines state that the MitraClip should only be considered for patients with chronic primary MR who remain severely symptomatic with NYHA class III to IV HF symptoms despite optimal heart failure ther- apy and who are considered inoperable [6].
The ESC guidelines recommend that MitraClip may be considered in patients with symptomatic severe primary MR who fulfill the echo criteria of eligibility, are judged inoperable or at high surgical risk by a ‘heart team,’ and have a life expectancy greater than 1 year [7].
Figure 4: Clip
Furthermore, based on experience the EVEREST trials and from observational studies, ESC guidelines suggest that MitraClip is feasible at low procedural risk in patients with secondary MR in the absence of se- vere tethering and may provide short-term improve- ment in functional condition and LV function [7].
While the AHA/ACC guidelines acknowledge that MitraClip provides a less invasive alternative to sur- gery, it is noted that the procedure is not yet ap- proved for clinical use in the United States [6].
The COAPT trial is a currently enrolling, random- ized, parallel-controlled, multicenter clinical eval- uation of the MitraClip device for the treatment of clinically significant functional mitral regurgitation in symptomatic heart failure subjects who are treated per standard of care and who have been deemed in- eligible for mitral valve surgery. Eligible subjects will be randomized in a 1:1 ratio to the MitraClip device or to no MitraClip device (control group). The primary outcome measures include the primary safety end- point (composite of single leaflet device attachment, device embolizations, endocarditis requiring surgery, mitral stenosis requiring surgery, and any device re- lated complications requiring non-elective cardiovas- cular surgery) and the primary effectiveness (recur- rent heart failure hospitalizations). The results of this study are eagerly anticipated to prove the efficacy of MitraClip in patients with functional MR.
Sharma, R.A. et al.
An Overview of the MitraClip Procedure